LEGIONELLA PNEUMOPHILA - FAQ
Influence of preservatives in urine on Legionella detection with Coris K-SeT product
The Sodium Borate & Formate preservatives or additive mix (chlorhexadine, ethyl paraben and sodium propionate) present in urine collection tubes (Greiner Vacuette® and BD Vacutainer® ) do not induce any background on the reactive membrane of Coris Legionella K-SeT kit. Furthermore, analytical sensitivity of Coris Legionella kit is similar when the dilution threshold (ie., the last detected dilution of a positive Legionella control) was performed in the presence or absence of these preservatives. In conclusion, there is no restriction for the use of BD and Greiner tubes with Coris Legionella K-SeT (# K-1215, K-1515) for the diagnostic of legionellosis in urine.
What immunoassay kits are validated with antigens?
Is the buffer the same for all Coris BioConcept kits? If I need some more for my tests, may I use the one from another kit?
According to the "Instructions For Use", it is not allowed to exchange buffer between kits. If you have volume problems, please contact your distributor who is able to provide additional vials. Nevertheless, the buffer is the same within one product of different lot numbers (e.g. Rota-Strip buffers exchange between different lots will not give rise to any problems on the strips).
At present, 8 different kinds of buffers are used in Coris’products, i.e., RE-A Buffer (for Influ –A&B Respi K-SeT), Extraction Buffer (for RSV-Strip), HC Dilution buffer (for Adeno Respi-Strip, Combi-Strip, Pylori-Strip, GastroVir-Strip, for Crypto-Giardia Duo-Strip, Giardia-Strip, and all cassette formats of the corresponding kits), HydroK buffer (for dry swab Test restricted to AdenoRespi K-SeT), ST-A buffer (for C.diff-Strip and Clostridium K-SeT), LY-A Buffer (for RSV Respi K-SeT and for kits RESIST), BL-A Buffer (for HAT Sero K-SeT), ST-1/ST-2 Buffers (for Strep-A Respi-Strip), and Sample Dilution Buffer for all the other products.
Regarding the GastroVir Kit we received some particular comments claiming that the Control Line was too faint. The buffer has been improved to allow a better migration of the latex microspheres leading to a better and a more homogeneous signal. This improvement does not affect any characteristics of the kit (i.e. specificity and sensitivity).
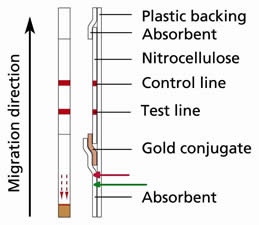
When dipping the stick in diluted sample, the level of sample passes beyond the limit indicated by the arrows at the bottom of the stick. Is there any consequences on the test reactivity?
The red arrow indicates the limit that could not be exceeded beyond during any incubation.
It could happen that in some cases operator exceeds the indicated limit line by using different but routinely used tubes in laboratories. However, it's still possible to security a validate test as long as the incubation level is still below 1cm (i.e. the green line indicated on the figure) while the gold conjugate is placed at 2cm from the bottom of the stick.
What happens when clinical specimen is a strongly positive specimen? What about the control line?
There are two different kinds of conjugates – a specific reagent for test line and a specific reagent for the control line - in the strip. A strong positive specimen has NO effect on the intensity of the control line onto the nitrocellulose of the strip. As long as the control line appears - with whatever intensity level - the test should be regarded as validated.
Why does the control line sometimes appear very weak while the test line is positive?
Control line is aimed to ensure that chromatography has been performed up to the top of the strip. It is not related with the result of the diagnostic itself.
Sometimes, the control line might be "slightly weak "without any precise reason. It could be only an effect of the sample migration and / or responsiveness at the control line. To sum up, the control line is aimed to validate the test using various specimen exists.
Why is stability relatively short after opening of bottle containing strips?
The product is stable during at least 15 weeks after first opening. 15 weeks is quite high: that means at least 2 tests can be performed per week. Stability depends on the storage conditions.
Immunochromatographic test uses nitrocellulose support which is sensitive to humidity.
That's why the desiccant is recommended to be kept in the tube and in the cap.
In each case, there is an internal control (upper line). This internal control will appear if the test is running well. However the test could be used after 15 or 20 weeks and the result can be valid if the control line appears.
How to use correctly Coris BioConcept's kits?
- Always store the reagents at the indicated storage temperature : between 4 and 30°C for all kits except for Oligochromatographic kits which contain Ampli-Reagents that requires to store at –20°C
- Always use gloves manipulate kit components and samples
- Always use the provided (Extraction, Dilution, …) buffer even for the sample already diluted (e.g. in a transport medium)
- Always ensure that the solution sample – buffer is homogenous before testing
- Always ensure the sample - buffer immerse below the limit line marked by red arrow on the strip to avoid colloidal gold conjugate dilution in the buffer
- Always read the results when the stick is wet (between 10 and 15 minutes depending on the test)
- After opening of the tube which contains the strips, quality is guaranteed during 15 weeks
- Always close the tube after every use to avoid that strips become wet. Always leave the desiccant inside the tube. If storing the strips' tube at 4°C, always ensure to warm up to room temperature before proceeding with the test
How shall we use positive controls with our kits?
The positive control should be used and diluted with the provided buffer of the respective kit. Then directly plunge the strip into the dilution. The mixture should be well homogenized before testing. Use the mixture if necessary and follow kit instructions.
How many tests can be performed with a positive control?
It is various between positive controls forms, e.g. freeze-dried or liquid.
The dilution level of positive control determines the number of possible tests. Please reference it to the Instruction For Use of each positive control.
The bottom of the nitrocellulose of the strip shows a pinkish aspect. Is it normal?
No. Maybe the sticker (with arrows) covering the bottom of the nitrocellulose is peeled off. In this case, the test is invalidated. Please perform the test with another strip.
The liquid does not go up the strip.
If the sample does not migrate due to the presence of particles, centrifuge the diluted sample. Collect the culture supernatant and perform the test again.
Is it a problem if the test has "run" more than the recommended time?
Yes. It might cause the appearance of false positive signal on test.
The signal of the test line is very weak in comparison with the control line. Do I have to consider that the result is positive?
The control line is aimed to ensure the user that the chromatography is performed up to the top of the strip. It does not give any indication on the diagnostic result. Whenever a signal is observed on the test line (weak or strong), it means that the sample is positive for the pathogen of interest.