Our Contract Development and Manufacturing Organization offering
What we do
At Coris BioConcept, we offer a full-service CDMO platform tailored to in-vitro diagnostics: from development through pilot production to commercial launch. Leveraging over two decades of expertise in rapid tests (immunochromatography, electrochemistry, LAMP (isothermal amplification), ELISA) and our newly strengthened molecular portfolio, we partner with innovators worldwide to bring their assay ideas to life. Sample types may be blood, plasma/serum, urine, respiratory samples, stool, saliva, crust/pustule, bacterial cultures (colonies or liquid), blood culture,...
What we offer
Coris BioConcept offers a broad range of services from concept feasibility to manufacturing, including prototype development, industrialization and regulatory support.
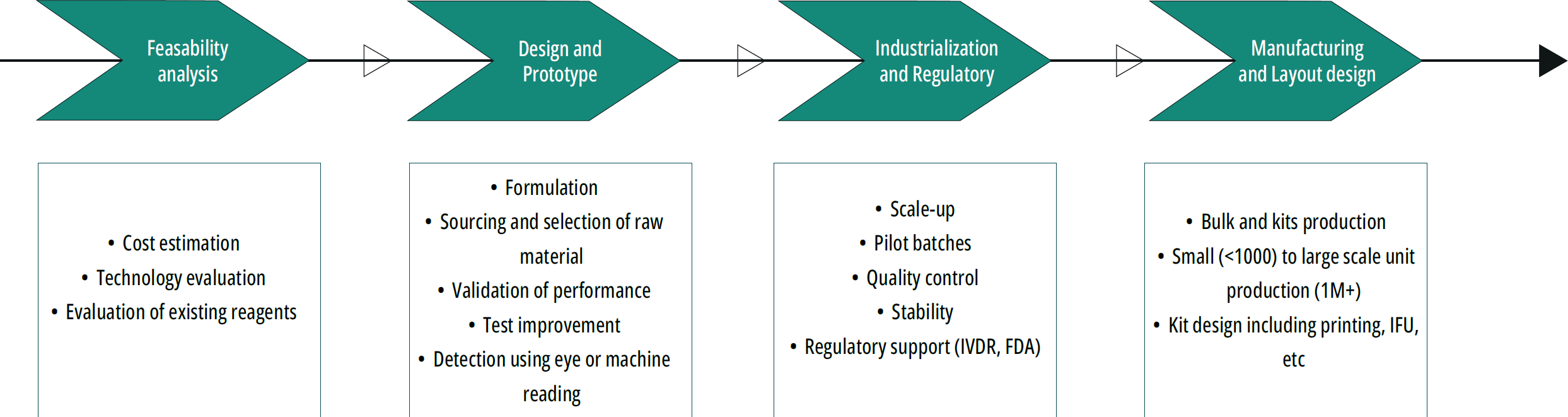
- In-depth expertise: our team masters all phases of diagnostic assay development, leveraging a full range of skills to meet your objectives.
- Flexibility: we start from your objectives and build around them, ensuring every solution fits your project perfectly.
- Cost-effectiveness: being a SME helps us to significantly reduce development time and cost.
- Reliability: we have been selling our own tests since last century across the world, guided by a strict quality management system (ISO 13485:2016), and with a focus on robustness and reproducibility. Our commitment and the quality of the service provided has led us to become a long-term service provider to several companies (up to 8 projects for a unique company).
- Intellectual Property: protecting both of our proprietary data is our top priority. We operate under strict confidentiality protocols, and by keeping all developments in-house at our Belgian facility with a dedicated team, we ensure your IP remains fully safeguarded.